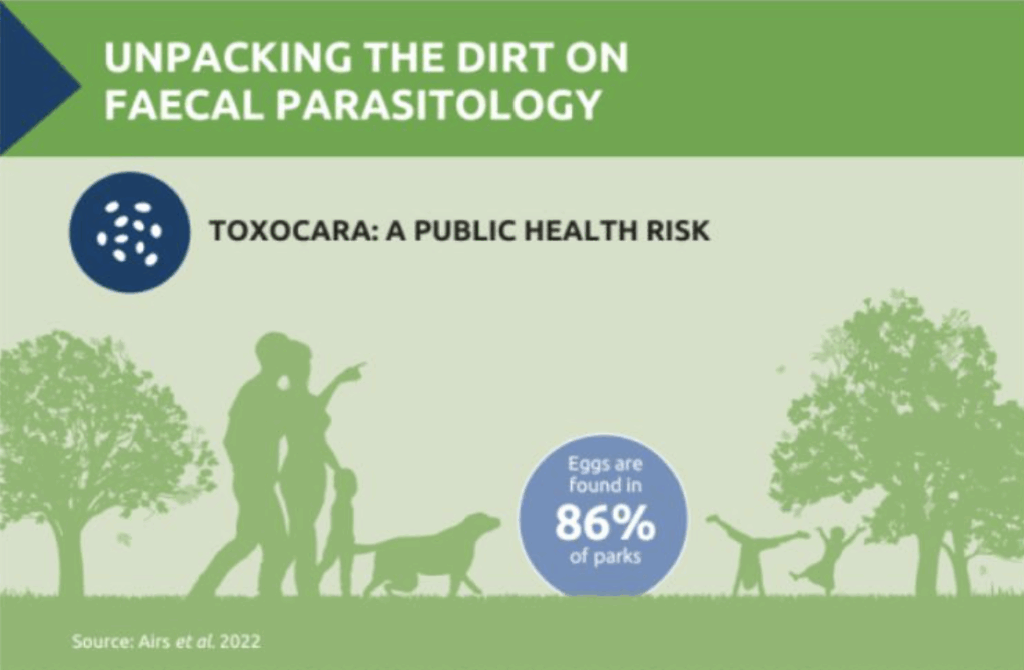
Unpacking the Dirt on Faecal Parasitology
Author: Jana Leontescu DVM, MRCVS
Parasite control has played a vital role in companion animal preventative health care over the last few decades. However, increasing concerns over the environmental impact of anthelmintic drugs and emerging signs of resistance are prompting a shift in clinical approach.1,2
Inspired by similar moves in antimicrobial stewardship, the focus is turning towards risk-based parasite control, supported by diagnostic testing. Testing enables clinicians to identify infections with confidence, target treatment where it is genuinely needed, and help safeguard both animal and public health through more sustainable prescribing.
Resistance, risk and the role of testing
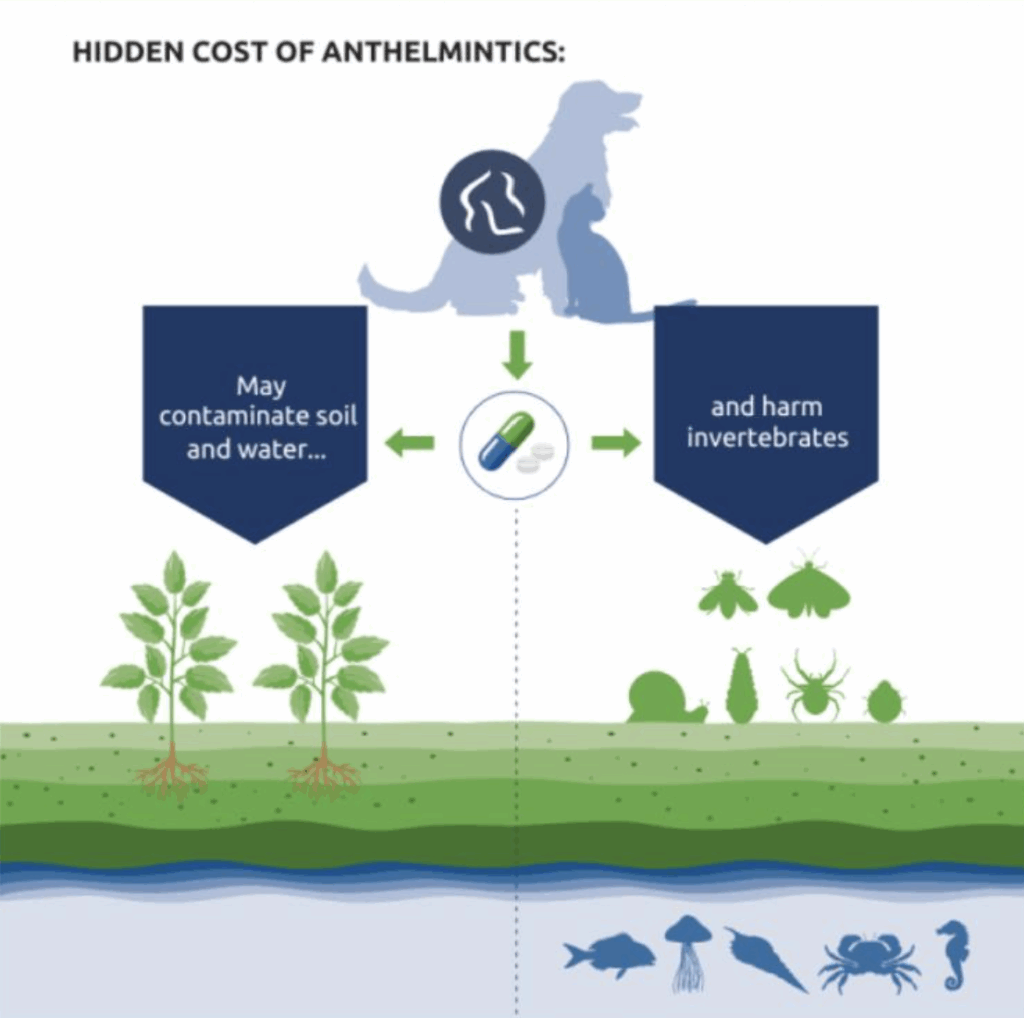
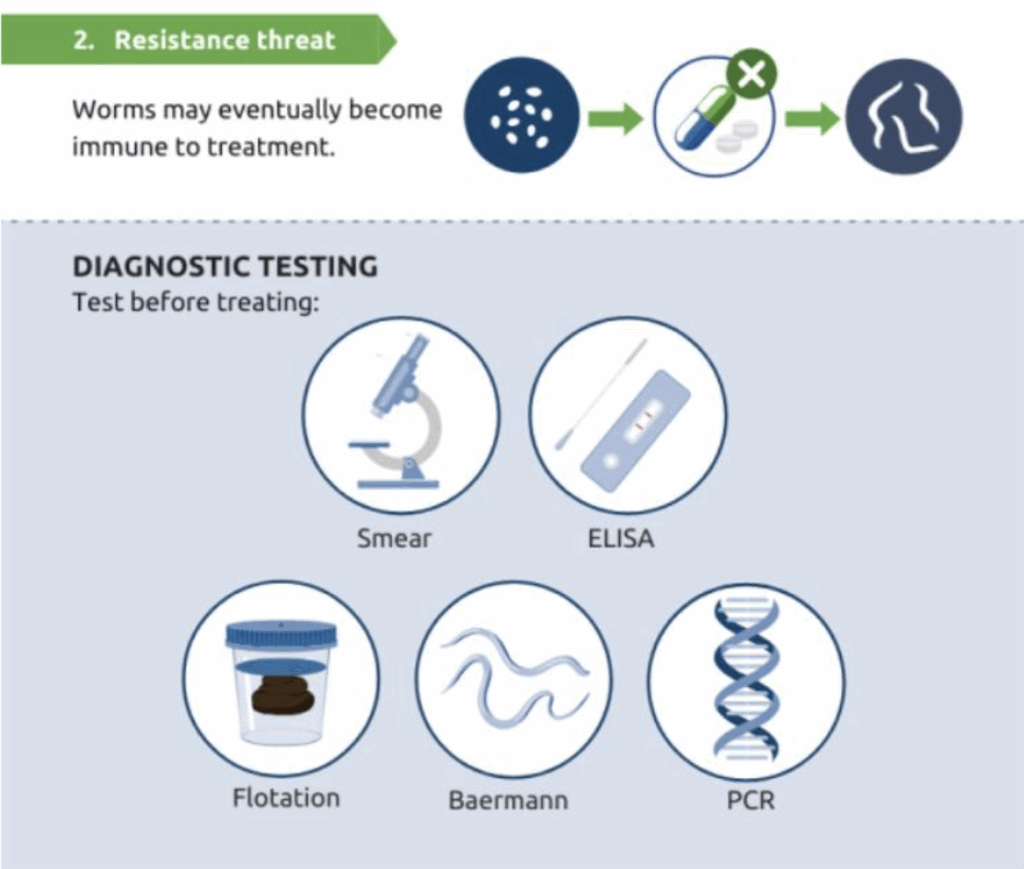
While anthelmintics remain a cornerstone of parasite control, increasing attention is being paid to the potential consequences of overuse. Resistance in companion animal parasites may not yet be widespread, but early warning signs are emerging and resistant strains of Ancylostoma caninum (hookworm) have already been documented in North America.
Beyond resistance, environmental contamination is another key concern. Studies have shown that active ingredients in parasiticides can persist in soil and waterways, potentially harming non-target species, especially invertebrates.
At the same time, parasites like Toxocara spp. pose a significant public health risk. Dogs and cats can shed thousands of eggs into the environment, and in the UK, approximately 86% of parks have tested positive for Toxocara contamination.3 There is a pressing need to balance effective parasite control with environmental responsibility and public health responsibility – diagnostic testing forms a key part of that solution.
The diagnostic toolkit
Faecal parasitology involves the identification of parasite eggs, cysts, oocysts or larvae in faecal material. A variety of in-house and laboratory-based tests are available, with some detecting a broad range of organisms and others offering species-specific results.
As with any diagnostic investigation, a thorough history and clinical examination remain essential. These initial findings provide valuable context for interpreting results and help to guide test selection – whether pointing towards a specific parasite or indicating the need for a broader testing panel. They also contribute to forming a more complete clinical picture, supporting accurate diagnosis and prognosis.
Faecal smear
Faecal smears are a quick and cost-effective way of identifying parasites in a faecal sample. This can be done in-house and is useful in identifying motile protozoa, such as Giardia (Figure 1).
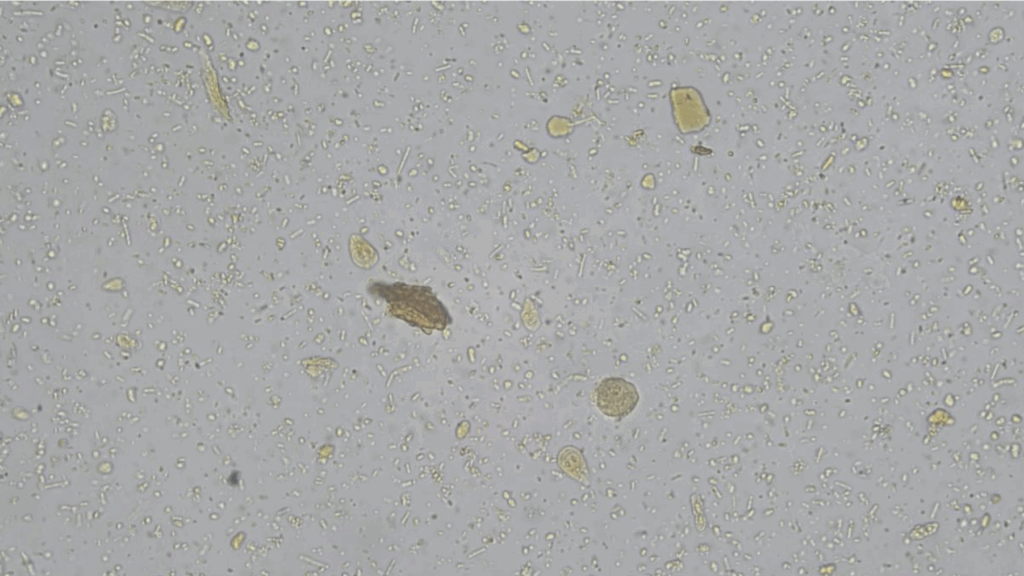
Further processing of faecal smear slides through staining can identify agents such as Cryptosporidium. However, sensitivity is limited, especially when parasite load is low.4 Smear interpretation is also highly dependent on user experience and time availability and where required, submitting samples to an external laboratory may improve diagnostic accuracy.
ELISA
Enzyme-linked immunosorbent assays (ELISA) can be performed using specific in-house SNAP tests or can be submitted for external laboratory testing. Faecal ELISA tests detect parasite antigens present in the faeces and results are either positive or negative. In-house antigen SNAP tests available for Giardia have been found to have good sensitivity and specificity.5 ELISA technology is also commonly used to detect Cryptosporidium in faeces. However, it is important to note that ELISA is not suitable for monitoring treatment success in either Giardia or
Cryptosporidium infections, as antigen may remain detectable in the faeces even after the infection has resolved.
Faecal flotation
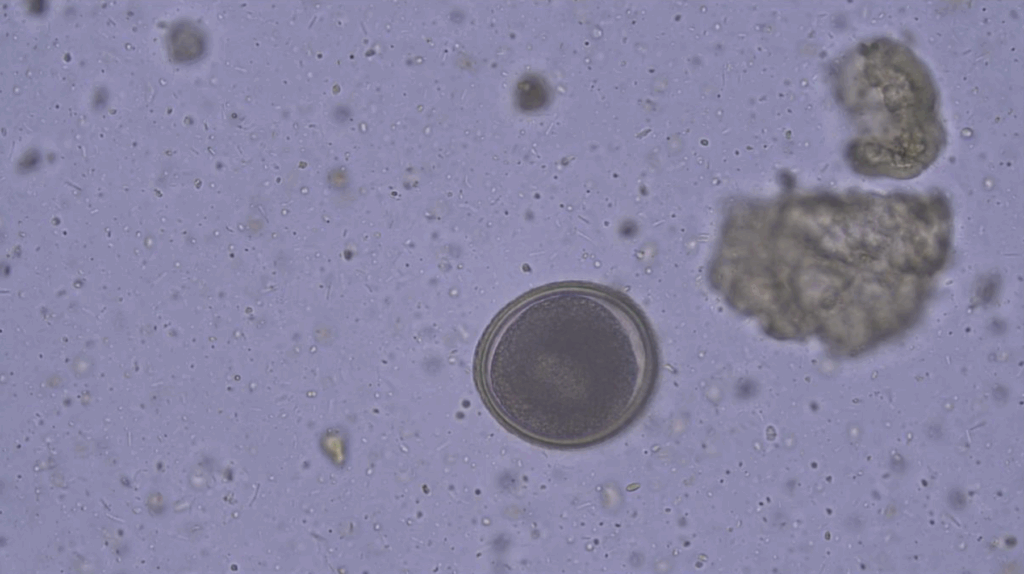
This technique utilises the different specific gravity of different parasite stages to separate them from faecal debris. The recovered material is then examined microscopically. This test can be performed in a qualitative and/or quantitative manner, however, the latter is more common for large animal species. Zinc sulphate solution is commonly used to identify Giardia cysts, whereas sodium nitrate is used to identify nematode eggs. The resulting solution is then examined under a microscope. This diagnostic test can identify the following parasites:
Nematode eggs
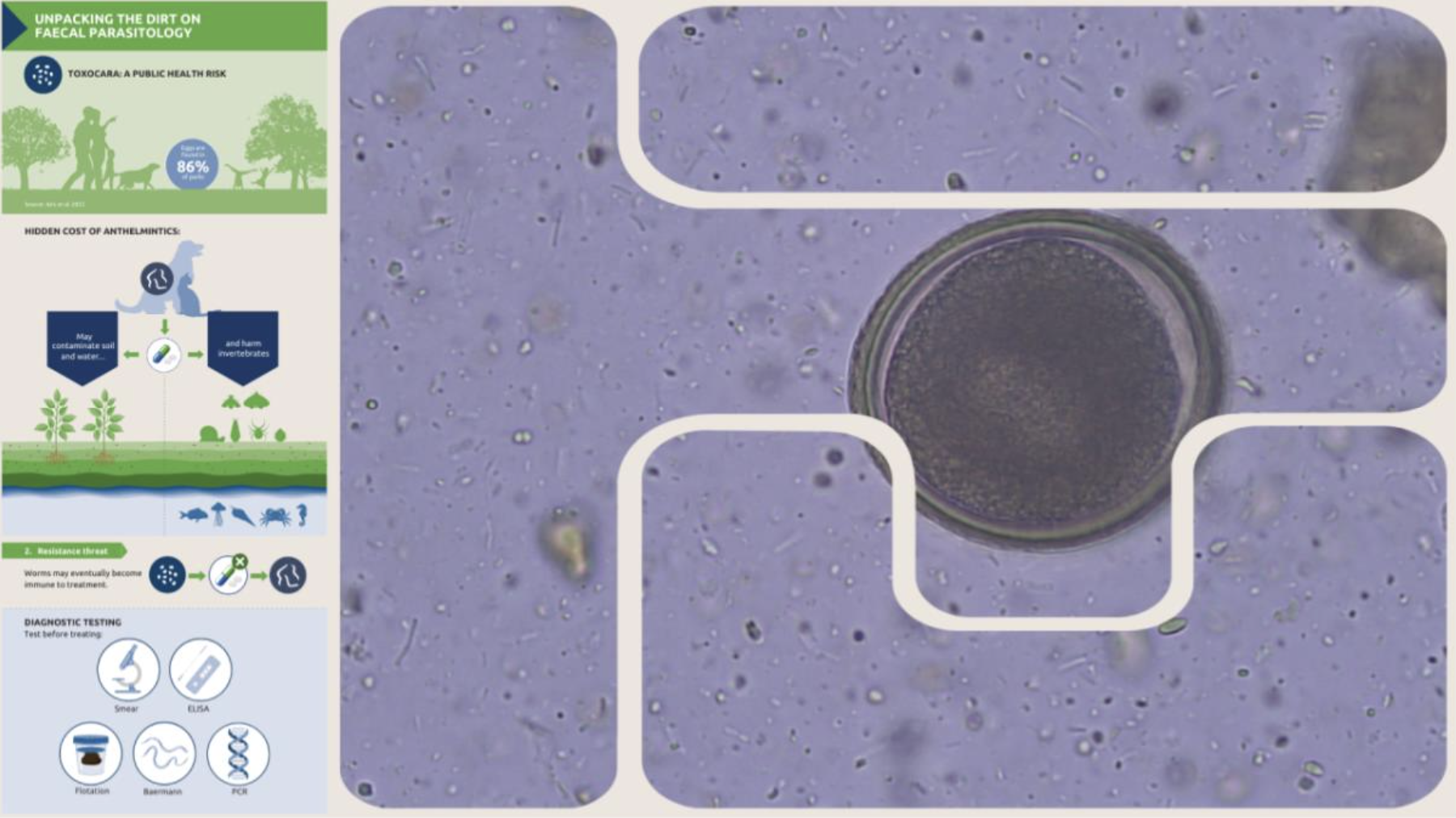
o Roundworm e.g. Toxocara spp. (Figure 2), Toxascaris spp.
o Whipworm e.g. Trichuris spp.
o Hookworm e.g. Ancylostoma spp, Uncinaria spp.
Cestode eggs e.g. Diplylidium spp. (Figure 3)
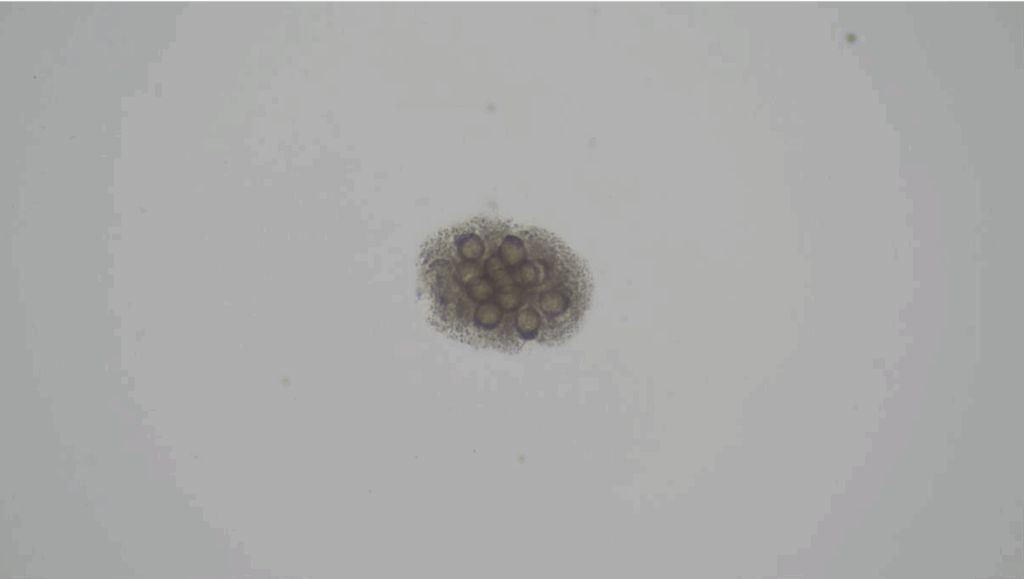
Coccidial oocysts e.g. Cystoisospora spp. (formerly Isospora)
Protozoal cysts e.g. Giardia
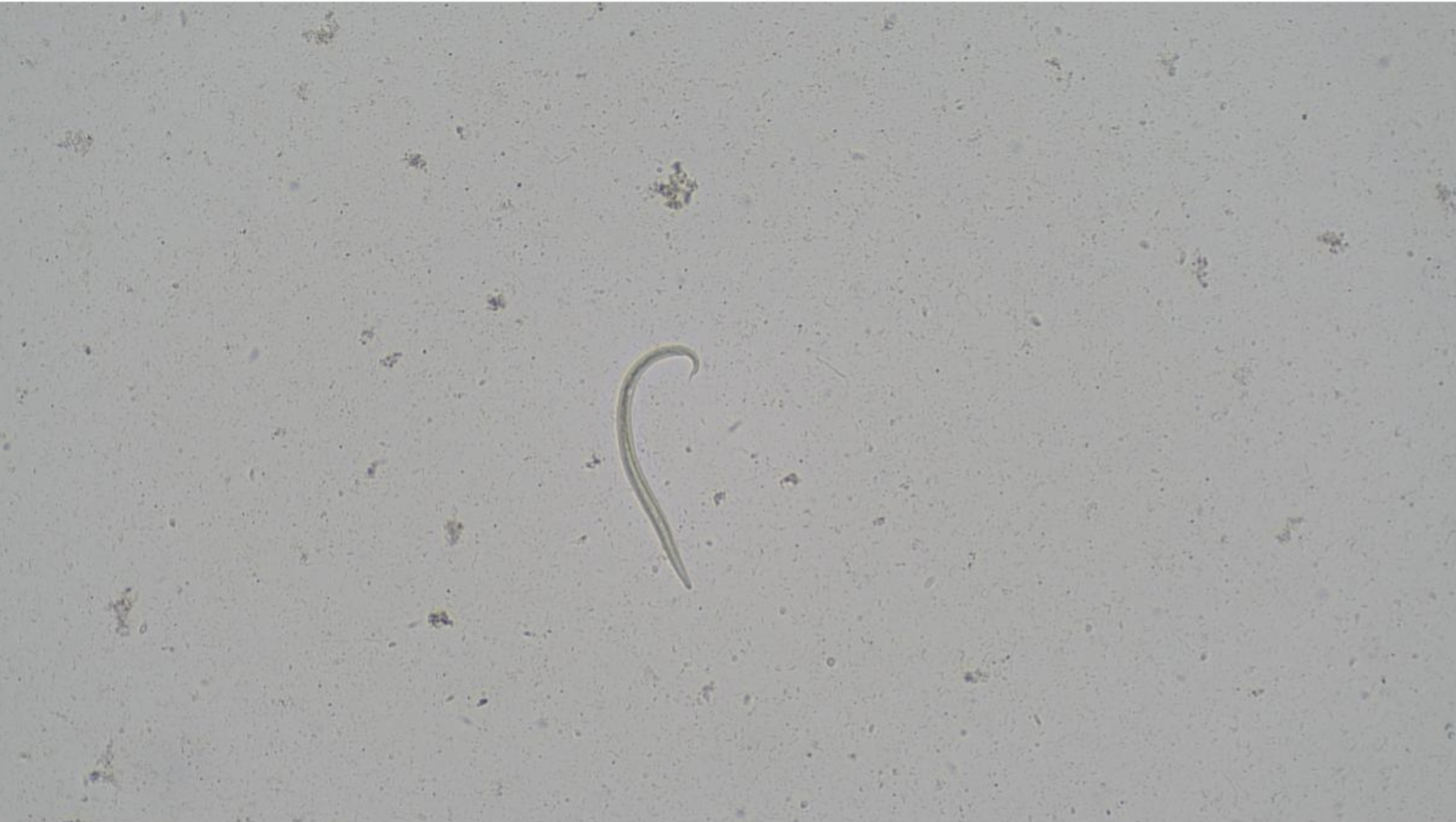
Baermann technique
This technique is used to detect first stage lungworm larvae (L1) in faecal samples, including Angiostrongylus vasorum (Figure 4) in dogs and Aelurostrongylus abstrusus in cats. It is non-invasive, relatively simple to perform and remains a widely used method of diagnosis.6 However, intermittent larval shedding can limit test sensitivity. Collecting and pooling faecal samples over three consecutive days can help improve diagnostic yield. In practice, many clinicians now favour blood-based antigen testing for A. vasorum, which offers rapid, reliable results and is less affected by the timing of larval shedding. It is worth noting that antigen testing is not available for A. abstrusis.
PCR
Faecal panels often employ PCR technology to simultaneously detect multiple pathogens from a single sample. These multiplex assays vary between laboratories but typically include combinations of parasitic, bacterial and occasionally viral targets. They are particularly valuable in cases presenting with non-specific gastrointestinal signs, in multi-pet households, or in animals with known exposure risks.
With growing concerns around resistance and environmental impact, there is a clear need to move beyond routine blanket worming. Clinicians should aim to educate owners on the benefits of diagnostic testing and support the move away from blanket therapy into risk assessment-based control.7 In doing so, we can help preserve the effectiveness of anthelmintics while promoting better outcomes for pets, people and the planet.
About the author
Jana Leontescu DVM, MRCVS is a qualified veterinary surgeon with a background in clinical practice in both Romania and the UK. Her interest in veterinary science led her to transition from practice to diagnostic pathology, joining NationWide Laboratories as part of the microbiology team with a focus on parasitology. Now training as a Clinical Pathologist, she is working towards board certification, expanding her expertise in laboratory diagnostics across a range of disciplines. Her experience on both sides of veterinary medicine gives her a well-rounded perspective on the challenges of diagnosing and managing disease.
References
1. Little, C.J. & Boxall, A.B. (2020), Environmental pollution from pet parasiticides. Veterinary Record, 186: 97-97. https://doi.org/10.1136/vr.m110
2. von Samson-Himmelstjerna, G. et al. (2021). Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses – Yet it is
of major concern. Int J Parasitol Drugs Drug Resist. 17:36-45. doi: 10.1016/j.ijpddr.2021.07.003. Epub 2021 Jul 25. PMID: 34343829; PMCID: PMC8347694.
3. Airs, P. et al. (2022). WormWatch: Park soil surveillance reveals extensive Toxocara contamination across the UK and Ireland. Veterinary Record. 192. 10.1002/vetr.2341.
4. WCVM. (2025) Learn about parasites: Diagnostics. Available online: https://wcvm.usask.ca/learnaboutparasites/diagnostics/faecal-direct-smear.php [Accessed: 10/7/25]
5. Olson, M.E., Leonard, N.J. & Strout, J. (2010) Prevalence and diagnosis of Giardia infection in dogs and cats using a fecal antigen test and fecal smear. Can Vet J.51(6):640-2. PMID: 20808578; PMCID: PMC2871365.
6. Oehm, A.W. & Schnyder, M. (2022) Adult parasite burden and excretion of first-stage larvae of Angiostrongylus vasorum in dogs: Methodologically relevant diagnostic aspects and associations with serological detection of parasite antigen and specific antibodies. Veterinary Parasitology, Volume 312, 109814, ISSN 0304-4017, https://doi.org/10.1016/j.vetpar.2022.109814.
7. Bagster, A. & Elsheikha, H. (2022) Perception of UK companion animal veterinarians on risk assessment based parasite control. Vet Parasitol Reg Stud Reports. 34:100774. doi: 10.1016/j.vprsr.2022.100774. Epub 2022 Aug 8. PMID: 36041809.
Original publication: Veterinary Edge, issue 55, September 2025, pp 28-30


